Services for
Geothermal & Oil&Gas
Membrane Nitrogen Injection
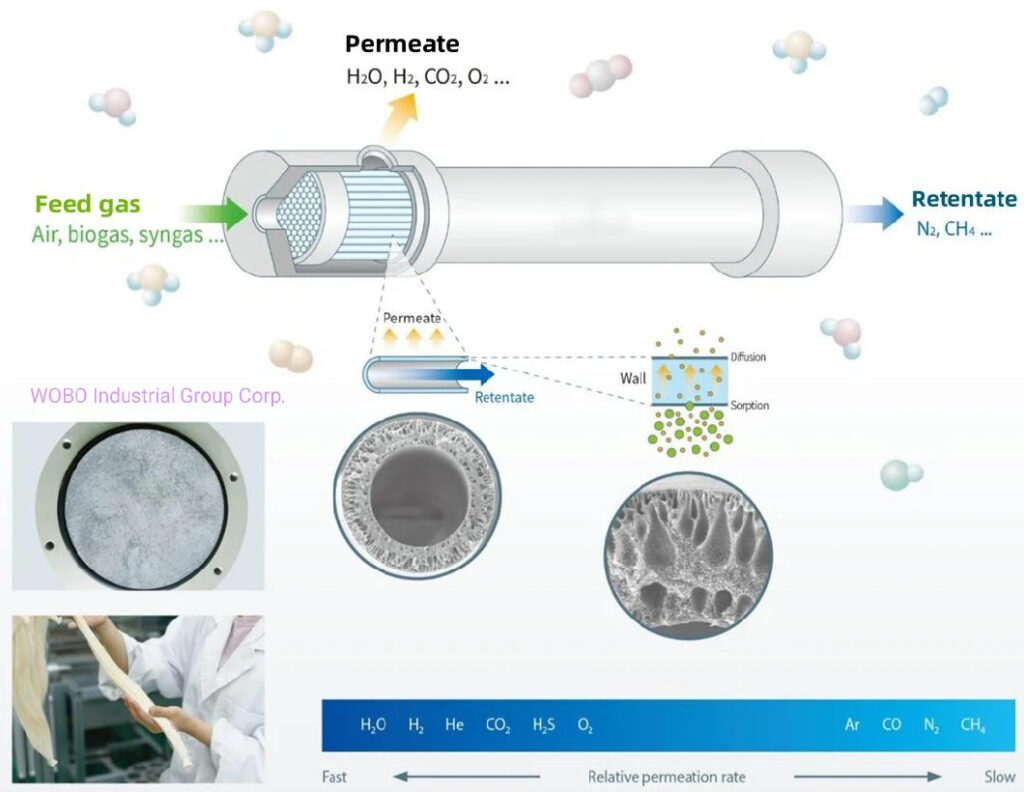
Membrane modules contain over a million fibers. Compressed feed air is passed down the bores of the fibers at one end of the module, with enriched nitrogen product gas exiting from the opposite end. Oxygen and water vapor are selectively removed and vented from the feed air as it flows through the module.
Specifically designed for rapid deployment to remote locations. Especially beneficial over cryogenic systems for remote and logistically challenging locations where the delivery of liquid nitrogen is often too difficult and costly. The losses and hazards associated with the storage, transport and handling of cryogenic liquids do not apply to membrane nitrogen systems.
How Nitrogen Membranes work
The air that we breathe contains approximately 78% nitrogen, 21% oxygen and 1% other gases, such as Argon and Water Vapour. Membrane Nitrogen systems use this unlimited supply of the raw materials to produce specific purities of nitrogen. Selective permeation is the general principle behind a membrane system. Each gas has a characteristic permeation rate that is a function of its ability to dissolve and diffuse through a membrane. This characteristic allows “fast gases”, such as oxygen, to be separated from “slow gases” such as nitrogen. The driving force of the separation process is the differential pressure created between the (compressed) feed air side and the low pressure side of the membranes.
The actual generation of nitrogen takes place in the membrane separator. Each separator consists of a bundle of millions of hollow fibre membranes in a cylindrical shell, arranged much like a shell and tube heat exchanger. The compressed air is fed into the inlet end of the separator and flows inside the hollow fibres towards the opposite end. On the way the air molecules start to permeate through the walls of the fibres according to their permeability. Oxygen, carbon dioxide and water vapour permeate faster than nitrogen. The membrane units produce nitrogen with an atmospheric dew point of about -73 °C. Further dehumidification may be achieved by treating the compressed air with a desiccant dryer before it enters the membrane unit.
Applications of Membrane Nitrogen:
- Nitrogen gas lifting (“nitrogen capping”) of geothermal wells
- Nitrogen blanketing or gas lifting of oil and gas wells
- Nitrogen circulation of wells drilled underbalanced
- Nitrogen testing and blanketing of pipelines and process plants
- Immiscible gas stimulation of oil wells
- Any use of nitrogen in remote areas, where supply of bottled or liquid nitrogen is not feasible
What Purity Nitrogen can be Used for Inerting and for Preventing Oxygen Corrosion?
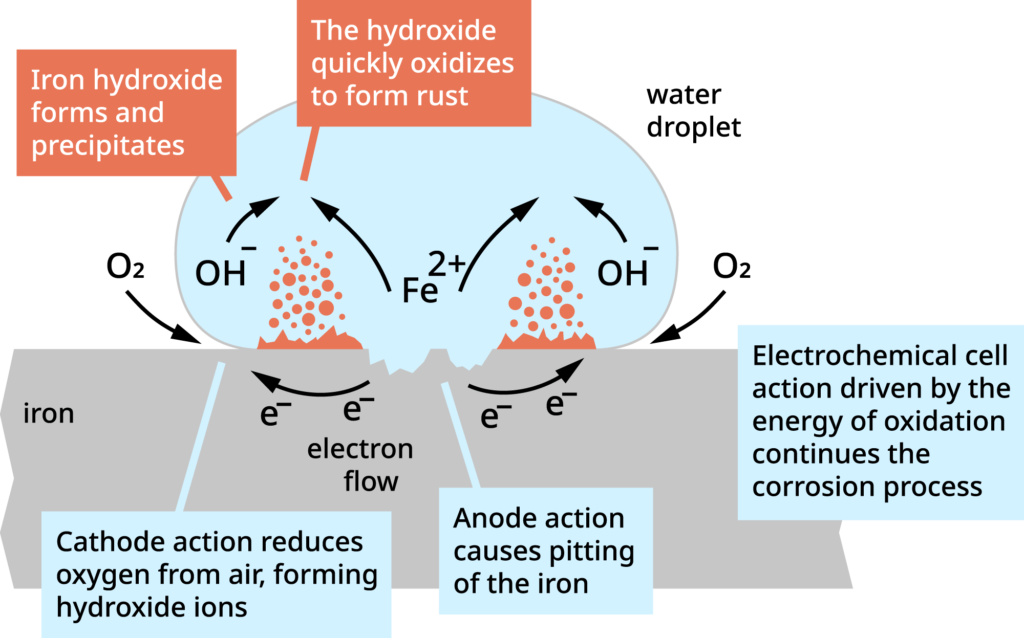
The corrosion of iron is an electrochemical process involving a chemical change of iron to iron oxide, and an electrical process involving current flow. This is shown in the picture.
- For this type of corrosion to occur all the following five factors must be present:
- Oxygen
- Electrolyte (moisture)
- Anode
- Cathode
- Metallic pathway
An obvious way to reduce or even eliminate oxygen corrosion is to blanket the metal (pipework, vessels etc) with an inert gas such as nitrogen. This also has the added benefit that it prevents flammable environments. The nitrogen can be supplied in very pure (typically 99.9+%) cryogenic form, however this can be very expensive in remote locations and when very large volumes are required. In such remote or high volume applications membrane units can be used to generate unlimited quantities of nitrogen on site and at purities ranging from 93-99%.
The membrane units run most efficiently at a purity of about 95%; as the purity increases the volume throughput is reduced considerably. Nitrogen purities >92% are outside the flammable area for a methane-oxygen-nitrogen mixture at any pressure and therefore can be used for inerting purposes.
In order to use 95% membrane-generated nitrogen to prevent oxygen corrosion we would need to eliminate one of the other four factors. The most practical way is to reduce the formation of electrolytes by removing moisture, and it just so happens that membrane units produce very dry nitrogen.

